Continuous Glucose Monitoring - Hope or Hype?
In the era of personal health optimization, tracking step counts, heart rate and sleep cycles has become routine. But recently, a new player has entered the field: continuous glucose monitoring (CGM). Imagine having the ability to see how every meal, workout, and even stressor impacts your blood sugar in real-time. Originally designed for diabetes management, CGM is now being embraced by health enthusiasts and self optimizers worldwide. But is this trend more than just hype?
How do Continuous Glucose Monitors work?
Continuous Glucose Monitors are devices that automatically track blood sugar levels in regular intervals, providing continuous data on your glucose levels, the body’s primary energy source. These devices use a tiny sensor inserted under the skin to measure glucose not directly in the bloodstream but in the interstitial fluid, the fluid surrounding your cells, sending the data to a smartphone for continuous tracking.
Originally developed over 20 years ago for diabetes management, CGMs are now marketed to non-diabetic users and have been readily accessible for individuals at modest cost.
CGMs as Tools for Health Optimization
Continuous Glucose Monitoring (CGM) technology promises a window into the dynamic world of glucose metabolism, providing data that can be leveraged in numerous ways to optimize health. By understanding how our bodies respond to different foods, activities, and stressors, we can make more informed decisions about our lifestyle choices.
Why is this important? Frequent spikes in blood glucose can indicate poor metabolic control, which is a significant risk factor for developing type 2 diabetes and associated chronic kidney disease. Moreover, high postprandial glucose levels are linked to an increased risk of cardiovascular diseases (CVD) as they contribute to endothelial dysfunction, inflammation, and atherosclerosis (Source).
This is important because even healthy individuals without diabetes and normal blood glucose levels still spend 30 minutes per day in a hyperglycemic (glucose too high) state and 15 minutes in a hypoglycemic (glucose too low) state (Source).
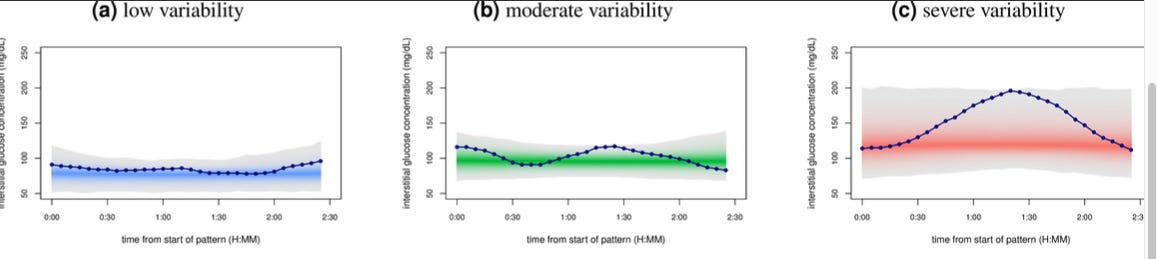
Glucotypes CGMs provide a detailed picture of your glucose levels throughout the day, revealing unique patterns known as “glucotypes” that intermittent prick tests could potentially miss. Using CGM, a study from Stanford University identified three main categories of "glucotypes" based on the intensity of glucose increase: low, moderate, and severe.
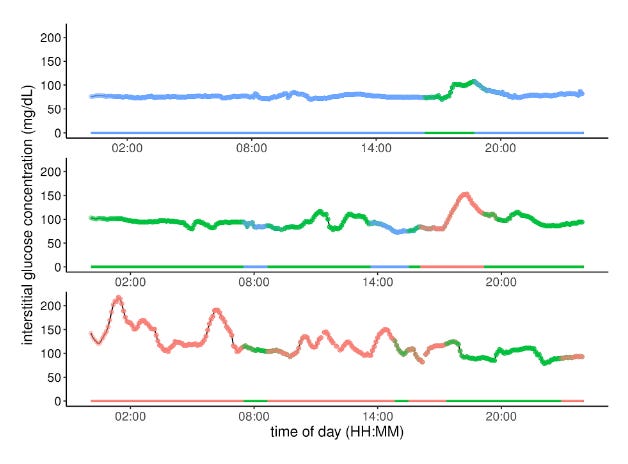
These “glucotypes” are dynamic and can change with dietary adjustments. For example, in a sub-study where participants alternated between different types of breakfasts, it was found that certain foods, like cornflakes with milk, caused significant spikes in blood sugar for most participants.
Surprisingly, over half of the participants who were previously deemed healthy experienced glucose spikes similar to those of prediabetic or diabetic individuals after consuming these meals (15% and 2% of the time, respectively)(Source).
This is highly relevant as continuous real-time data can significantly influence behavior, known as the "Hawthorne Effect." As shown by studies demonstrating improved self-management among diabetics (Source), immediate feedback from CGM can promote healthier dietary choices and lifestyle changes.
Whether glucotypes really offer a clinically useful tool in personalized precision health remains to be determined, as another study investigating glucotypes in two independent, large datasets did not find additional clinical insights from glucotypes when compared to mean CGM glucose (Source).
How might CGMs contribute to weight loss and personalized nutrition?
The theory is that by avoiding glucose spikes, individuals can better manage their insulin levels. When food is digested, glucose enters the bloodstream, prompting the pancreas to release insulin, which helps cells absorb glucose for energy. Excess glucose is converted to fat, so larger spikes can lead to more fat storage. Understanding which foods cause significant glucose spikes can help users make dietary adjustments to avoid them, potentially aiding in weight management.
While smaller randomized controlled trials could not yet definitely determine, if CGM-based personalized nutrition can improve metabolic health and outcomes in healthy (Source) or prediabetic and diabetic individuals (Source), in a recent observational study with a larger sample size the integration of CGM data, dietary logging, physical activity tracking, with personalized feedback based on general recommendations via a smartphone app led to significant improvements in metabolic health. Participants also adopted healthier eating habits, reducing daily caloric intake and improving their macronutrient profiles by increasing protein, fiber, and healthy fats relative to calories. As a consequence, participants showed reductions in blood glucose variability including hyper- and hypoglycemia, particularly among non-diabetics. Additionally, all groups experienced weight loss, with the most significant decreases seen in overweight or obese individuals (Source).
CGM for Diagnosis and Management of Diabetes and Prediabetes CGMs have been invaluable for diagnosing and managing diabetes for many years now. They allow for real-time adjustments to diet, medication, and lifestyle, improving glucose control and reducing complications. This has been demonstrated in numerous trials, including large randomized controlled trials (RCTs), which indicate that all of today's CGM systems enhance glycemic control and reduce the risk of hypoglycemia.
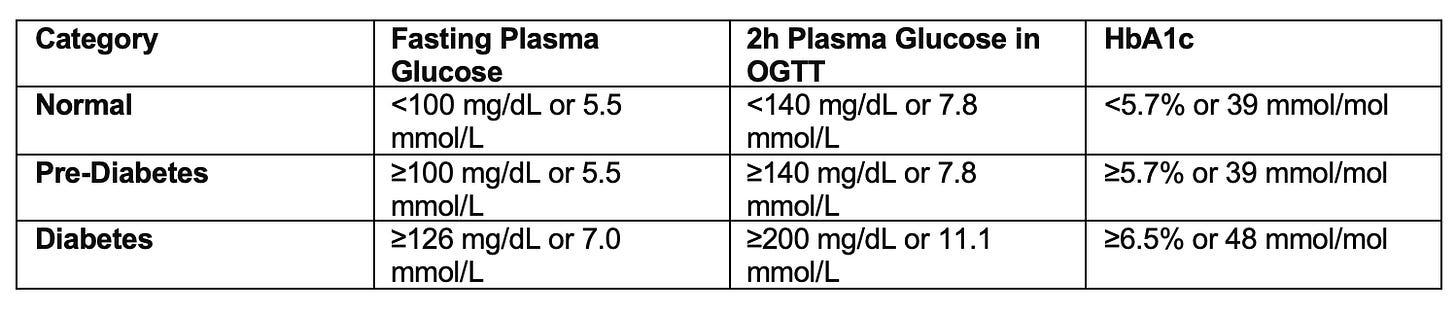
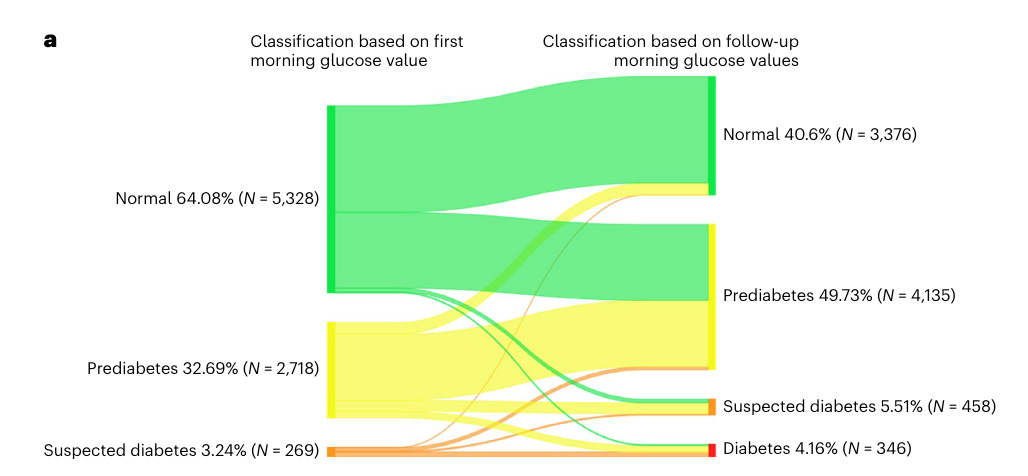
In addition, CGMs might help detecting prediabetes. Prediabetes is a symptom-free, “silent” condition and nearly 90% of individuals are unaware of the diagnosis (see box for diagnostic criteria).
Healthcare professionals show significant knowledge gaps in diagnostic and therapeutic strategies regarding prediabetes (Source) and current diagnostic approaches might miss a significant portion of patients with prediabetes. A recent study using continuous glucose monitoring (CGM) in adults aged 40-70 found that fasting glucose levels vary significantly day-to-day. Current plasma FG-based diagnostic criteria do not account for this variability, potentially leading to misclassification of glycemic status. This variability meant that 40% of individuals initially classified as having normal FG levels were reclassified as prediabetic, and 3% as diabetic based on sequential CGM measurements. These findings suggest that CGM data could improve the accuracy of diabetes diagnosis (Source).
Potential Pitfalls of CGM Use
While CGMs offer an exciting new, minimally invasive option in personal health optimization, we should consider limitations and pitfalls of CGM use.
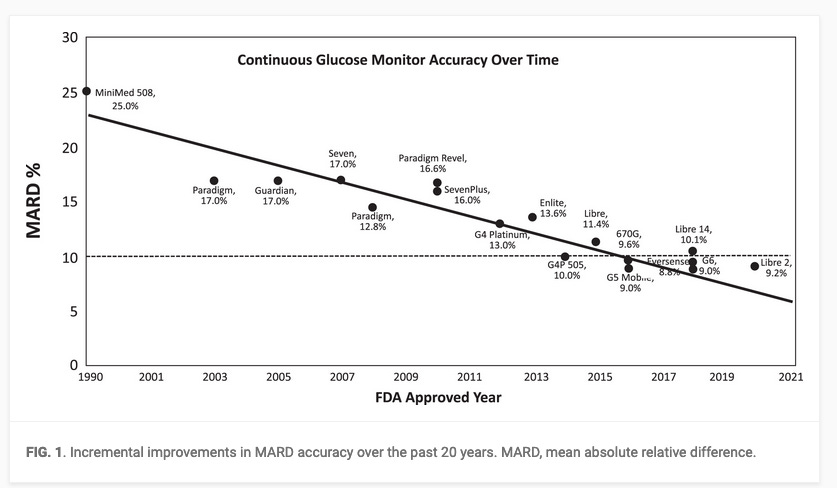
Accuracy: CGM technology has advanced, providing increasing accuracy sufficient for safe and effective diabetes management.
However, the regulatory bodies have less stringent requirements for integrated CGMs compared to blood-based glucose monitoring systems. For instance glucose levels are allowed to diverge up to 40 mg/dl from the actual plasma glucose levels (Source), which can result in different classifications of glycemic status within the same individual. Consequently, discrepancies between CGM and blood-based glucose measurements can significantly impact the diagnosis of diabetes or prediabetes,
Lag in Readings: CGMs measure glucose levels in the interstitial fluid rather than blood, resulting in a 5-20 minute lag behind real-time blood glucose levels. While this delay is crucial for diabetics needing to respond to sudden changes, it is less problematic for healthy individuals (Source).
Behavioral Impacts: Monitoring glucose levels can lead to healthier eating and exercise habits. However, there is also a risk of becoming overly focused on glucose levels, potentially leading to unnecessary anxiety or obsessive behavior regarding normal blood sugar fluctuations.
Complications: While CGMs are generally considered very safe, their minimally invasive nature can in principle lead to adverse events. A systematic analysis of 54 CGM studies found a complication rate of one event per eight weeks of use, with 98.5% of these being non-severe, primarily local skin reactions. In addition, most users reported less pain or discomfort with CGMs compared to traditional finger-prick glucose testing (Source).
Conclusion
While CGM technology offers unprecedented insights into our metabolic dynamics, its integration into everyday management of healthy, low-risk individuals warrants cautious consideration despite the favorable risk-benefit ratio. Despite exciting emerging data regarding the benefits of CGM, there is still no strong evidence that monitoring glucose levels improves health outcomes or aids in weight loss for healthy individuals. The benefits observed might be more related to increased health awareness rather than direct physiological impact.
How YEARS Does It
At YEARS, our evidence-based approach hinges on comprehensive diagnostics, blending cutting-edge technologies with established gold standards.
Continuous Glucose Monitoring (CGM) seamlessly integrates into this framework, offering real-time insights into metabolic dynamics serving as a patient-centered adjunct, enhancing our toolkit by providing continuous, real-time data that complements traditional testing methods.
By combining CGM with gold standard tests like fasting glucose, fasting insulin, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), and Oral Glucose Tolerance Test (OGTT), we achieve a nuanced understanding of your metabolic health. This fusion allows us to paint a holistic picture, identifying patterns and deviations crucial for personalized interventions.
How to Get Started
Getting involved is easy. Contact us through our website:
and visit our first clinic in Berlin to learn more about our comprehensive health assessment process. Our team is here to guide you through every step, ensuring a meaningful and impactful experience.
To health, vitality, and the years ahead,
The YEARS Team
References & Further Reading
Bailey, Timothy S., and Shridhara Alva. “Landscape of Continuous Glucose Monitoring (CGM) and Integrated CGM: Accuracy Considerations.” Diabetes Technology & Therapeutics 23, no. S3 (September 2021): S-5. https://doi.org/10.1089/dia.2021.0236.
Freckmann, Guido, Stefan Pleus, Mike Grady, Steven Setford, and Brian Levy. “Measures of Accuracy for Continuous Glucose Monitoring and Blood Glucose Monitoring Devices.” Journal of Diabetes Science and Technology 13, no. 3 (May 2019): 575–83. https://doi.org/10.1177/1932296818812062.
Hall, Heather, Dalia Perelman, Alessandra Breschi, Patricia Limcaoco, Ryan Kellogg, Tracey McLaughlin, and Michael Snyder. “Glucotypes Reveal New Patterns of Glucose Dysregulation.” PLOS Biology 16, no. 7 (July 24, 2018): e2005143. https://doi.org/10.1371/journal.pbio.2005143.
MD, Robert H. Shmerling. “Is Blood Sugar Monitoring without Diabetes Worthwhile?” Harvard Health, June 11, 2021. https://www.health.harvard.edu/blog/is-blood-sugar-monitoring-without-diabetes-worthwhile-202106112473.
O’Keefe, James H., and David S. H. Bell. “Postprandial Hyperglycemia/Hyperlipidemia (Postprandial Dysmetabolism) Is a Cardiovascular Risk Factor.” The American Journal of Cardiology 100, no. 5 (September 1, 2007): 899–904. https://doi.org/10.1016/j.amjcard.2007.03.107.
Shah, Viral N., Stephanie N. DuBose, Zoey Li, Roy W. Beck, Anne L. Peters, Ruth S. Weinstock, Davida Kruger, et al. “Continuous Glucose Monitoring Profiles in Healthy Nondiabetic Participants: A Multicenter Prospective Study.” The Journal of Clinical Endocrinology and Metabolism 104, no. 10 (October 1, 2019): 4356–64. https://doi.org/10.1210/jc.2018-02763.
Shilo, Smadar, Ayya Keshet, Hagai Rossman, Anastasia Godneva, Yeela Talmor-Barkan, Yaron Aviv, and Eran Segal. “Continuous Glucose Monitoring and Intrapersonal Variability in Fasting Glucose.” Nature Medicine, April 8, 2024, 1–8. https://doi.org/10.1038/s41591-024-02908-9.
Taylor, P. J., C. H. Thompson, N. D. Luscombe-Marsh, T. P. Wycherley, G. Wittert, G. D. Brinkworth, and I. Zajac. “Tolerability and Acceptability of Real-Time Continuous Glucose Monitoring and Its Impact on Diabetes Management Behaviours in Individuals with Type 2 Diabetes – A Pilot Study.” Diabetes Research and Clinical Practice 155 (September 1, 2019): 107814. https://doi.org/10.1016/j.diabres.2019.107814.
The Emerging Risk Factors Collaboration. “Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies.” The Lancet 375, no. 9733 (June 26, 2010): 2215–22. https://doi.org/10.1016/S0140-6736(10)60484-9.
References for Clinical Trials using CGM
Haak T, Hanaire H, Ajjan R, et al.: Use of flash glucose sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther. 2017;8:573–586.
Beck RW, Riddlesworth T, Ruedy K, et al.: Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317: 371–378.
Beck RW, Riddlesworth TD, Ruedy K, et al.: Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374.
Lind M, Polonsky W, Hirsch IB: Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317:379–387.
Heinemann L, Freckmann G, Faber-Heinemann G, et al.: Benefits of continuous glucose monitoring use in adults with type 1 diabetes and impaired hypoglycaemia awareness and/or severe hypoglycaemia treated with multiple daily insulin injections: results of the multicentre, randomised controlled HypoDE study. Lancet. 2018;391:1367–1377.
Haak T, Hanaire H, Ajjan R, et al.: Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55–73.
Tyndall V, Stimson RH, Zammitt NN, et al.: Marked improvement in HbA1c following commencement of flash glucose monitoring in people.
Charleer S, De Block C, Van Huffel L, et al.: Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care. 2020;43:389–397.
Charleer S, Mathieu C, Nobels F, et al.: Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: a real-world study. Clin Endocrinol Metab. 2018;103:1224–1232.
Wright EE, Kerr MSD, Reyes IJ, et al.: Use of Flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or non-insulin therapy. Diabetes Spectr. 2021;34:ds200069.
Kröger J, Fasching P, Hanaire H: Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279–291.