Imagine your car. When it was new, everything ran smoothly—the tires had perfect traction, the engine purred without a hitch, and the paint gleamed under the sun. However, as the years pass, the tires wear out, the engine might start having problems, scratches accumulate on the paint, and overall, it no longer resembles the car you once bought. The reason for this is simple: years of usage cause it to slowly break down — your car is aging.
You could spend a lot of money on new tires, a new engine, and touching up the paint from time to time. But none of these solutions will address the root cause of the problem.
What if you could work on the underlying issue itself, namely the aging of the car?
Aging and Its Risks
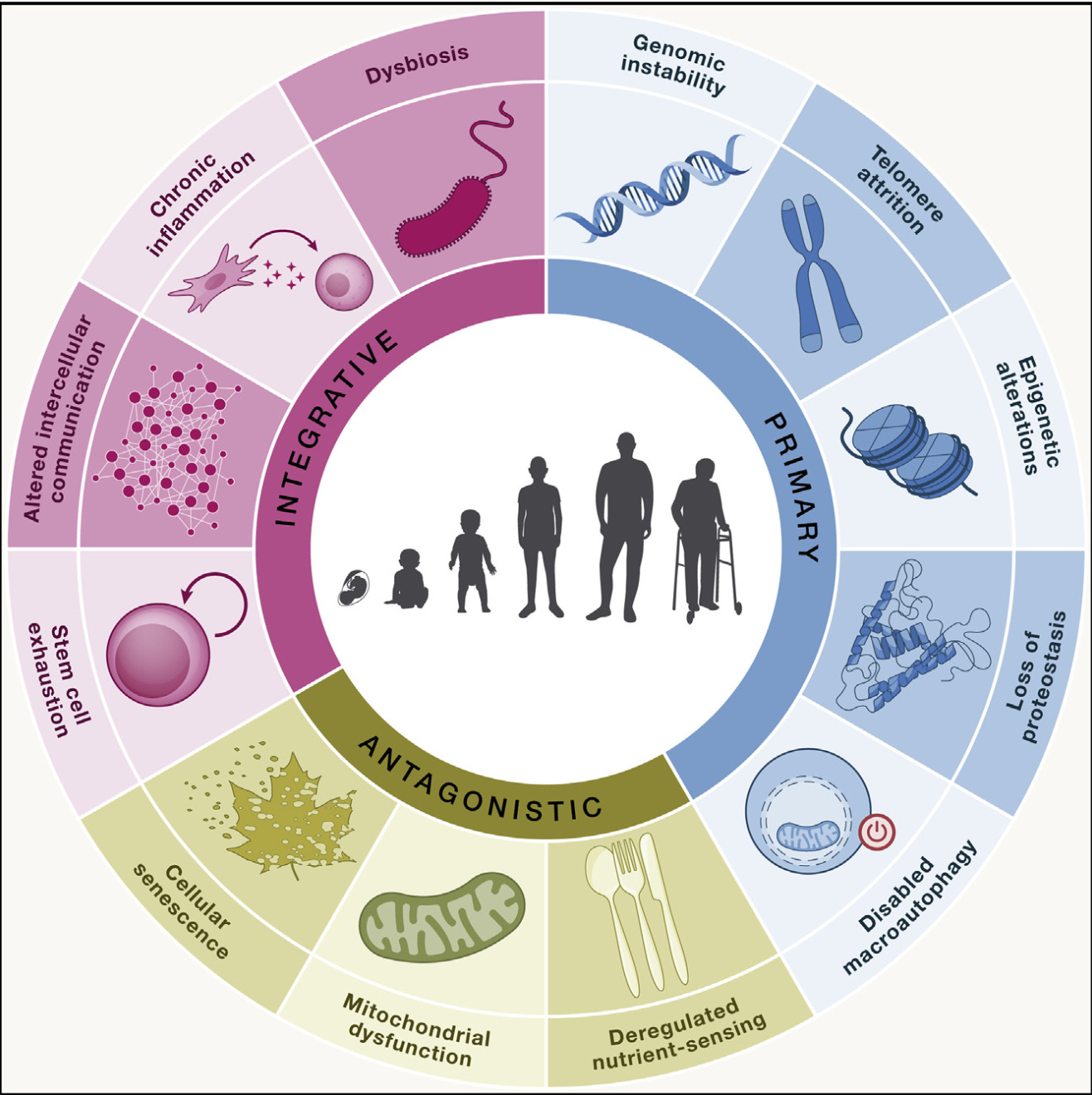
Let's shift focus and examine the human body. As we age, our biological systems also deteriorate, resulting in multiple changes across various organs (Aunan et al., 2016). Immune function declines, making us less capable of fighting infectious diseases promoting a chronic inflammatory response known as “inflammaging” (Ferrucci & Fabbri, 2018; Franceschi et al., 2018). This phenomenon contributes to multiple chronic diseases such as cardiovascular disease (e.g., heart attacks and stroke), chronic kidney disease, metabolic diseases, and neurodegenerative disorders (Furman et al., 2019). Furthermore, our muscle mass diminishes, a condition referred to as “sarcopenia”, impairing our strength and increasing the risk of falls (Larsson et al., 2019). Blood vessel walls thicken and stiffen (Fleg & Strait, 2012), leading to high blood pressure (Mattace-Raso et al., 2006). These are some of the functional changes that occur with aging. The underlying changes have been categorized into what are known as the hallmarks of aging (López-Otín et al., 2013). The original nine hallmarks include DNA instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication (López-Otín et al., 2013). Recent research has expanded this list to include chronic inflammation, loss of proteostasis, and dysbiosis, bringing the total to twelve hallmarks (Figure 1). These hallmarks are strongly interconnected and functionally related, which highlights the systemic and complex process of aging. They can be organized into a specific hierarchy. The so called primary hallmarks build up gradually and clearly drive the aging process (Gladyshev et al., 2021). Conversely, the antagonistic hallmarks have a more nuanced function, as they react to damage. When the accumulation of damage exceeds the compensatory abilities of both primary and antagonistic hallmarks, integrative hallmarks emerge, resulting in conditions like chronic inflammation (López-Otín et al., 2023).
The Health Implications
Consider your car once more: Just like your car, your body experiences a similar decline. With each passing year, the risks associated with aging intensify, establishing age as a dominant risk factor for a spectrum of conditions (Niccoli & Partridge, 2012), collectively termed age-related diseases (Maresova et al., 2019). These include major health concerns such as cancer (Hoeijmakers, 2009), cardiovascular diseases (Yan et al., 2021), Alzheimer’s disease (Hou et al., 2019), and diabetes (Fazeli et al., 2020). Furthermore, there is a strong correlation between advancing age and the prevalence of mental health conditions such as depressive disorders (Our World in Data, 2021). The impact of age on our overall health and well-being is inescapable.
Age does not only increase the likelihood of disease but also correlates with more years of life lost due to early death (Figure 2 and 3) (Our World in Data, 2019; Partridge et al., 2018) and more years spent living with illness or disability, known as disability-adjusted life years (DALYs) (Figure 4) (Partridge et al., 2018).
To put it bluntly: With age, you are more likely to die, and if you survive, you are more prone to disease, impacting the quality of your life. This not only places a significant burden on individuals but also on the healthcare system.
On top of that, this problem profoundly affects the health sector, as age-related diseases account for 51% of the overall global disease burden worldwide (Chang et al., 2019). With the population of individuals over 60 expected to double by 2050 (WHO, 2022), the urgency to address aging as a healthcare priority is undeniable.


Refocusing on the Underlying Risk
Age is the greatest risk factor for nearly every major cause of mortality in developed nations (Kaeberlein et al., 2015).
This statement emphasizes the need to expand our focus. Instead of exclusively focusing on diseases like Alzheimer’s, cardiovascular disease, and cancer, we should pay much more attention to the underlying risk factor: age.
The importance of considering aging as a risk factor has gained increasing attention. Even Dr. Francis Collins, former director of the National Institutes of Health, emphasized that in addition to studying each disease in isolation, it is equally important to investigate the underlying mechanisms of aging (Burch et al., 2014). The problem being, that in recent decades human lifespan has increased, yet healthspan has not. Our focus has been on treating individual diseases as they arise, thereby reducing the time to death, but not improving healthspan. Global lifespan increased to 73.4 years, while healthspan lagged behind at 63.7 years, as estimated by the WHO in 2019 (Ducharme, 2023; The Global Health Observatory, 2019). By targeting aging itself, we could not only reduce the adverse outcomes of age-associated diseases (Kaeberlein, 2013; Kaeberlein et al., 2015), but also increase healthspan. Healthspan refers to the period of life spent in good health, free from chronic diseases and disabilities of aging (Kaeberlein, 2018). The impact of targeting a single disease on healthspan would be minimal, as another chronic disease would soon take its place due to increased aging (Kaeberlein, 2019).
As illustrated in the graph below, for a typical 50-year-old woman with a life expectancy of 81 years, curing cancer would add approximately four years to her life expectancy. A similar outcome would occur with curing cardiovascular disease. Curing both would add a few more years, adding about 8 years. In complete contrast, slowing down aging is predicted to add more than 30 years of life expectancy, 25 of them being years in complete absence of chronic disease. On average, targeting aging results in 20 more healthy years than curing both cancer and cardiovascular disease.
Figure 5 (Kaeberlein, 2019): Slowing aging is more effective than curing disease. Displayed are the calculated impacts on life expectancy for a typical 50-year-old woman from curing cancer, heart disease, or both, relative to the impact of slowing aging. The figure was generated from data presented in Lombard et al. 2016. The coloring illustrates the hypothetical impact on health expectancy in each case, where green represents the absence of a comorbidity and the red represents a severe comorbidity.
We need to target biological aging directly, rather than employing a one-disease-at-a-time approach (Kaeberlein, 2019). This approach will both mitigate the outcomes of chronic diseases and, more importantly, add more healthy years to life.
The Emerging Field
The good news: Aging research, the scientific field actively looking into unraveling the underlying molecular mechanisms of age and aging, is advancing rapidly (López-Otín et al., 2023; A. T. Lu et al., 2023; Y. R. Lu et al., 2023; Meyer & Schumacher, 2024; Moqri et al., 2024; Wen et al., 2024). The importance of healthy aging and longevity is gaining more and more attention (Campisi et al., 2019). A pivotal shift has occurred as recent research indicates that biological age is modifiable. “Turning back time” might be possible (Mahmoudi et al., 2019).
Extensive research on lowering biological age has been conducted, encompassing a broad range of interventions (Guo et al., 2022). These include lifestyle interventions such as caloric restriction (Belsky et al., 2017; Waziry et al., 2023) the Mediterranean diet (Canudas et al., 2020) and exercise (Lohman et al., 2023; Sánchez-González et al., 2024). Pharmacological interventions range from SGLT-2 inhibitors (La Grotta et al., 2022; O’Keefe et al., 2023) and GLP-1 agonists (Chavda et al., 2024) to Rapamycin (D. J. W. Lee et al., 2024; Selvarani et al., 2020) and Metformin, which is currently being tested in a large six-year clinical trial (TAME - Targeting Aging with Metformin, 2024). Other approaches include thymus regeneration (Fahy et al., 2019), Senolytics such as Dasatinib and Quercetin (E. Lee et al., 2024) or approaches based on hormonal therapy (Jurić et al., 2020). But many studies have only been conducted on rodent models, and human data, particularly from large randomized controlled clinical trials, is currently limited.
Looking Ahead
Understanding aging as a novel risk factor is essential, but it raises several important questions. How should healthy aging medicine be approached? What are the practical considerations for establishing healthy aging clinics? What crucial principles need to be addressed? How do we measure aging? What interventions are supported by high-quality evidence?
If you are interested in exploring how best to measure aging and identify effective strategies for promoting healthy aging, stay tuned. Future posts will provide insights on measuring aging accurately, explain biomarkers of aging, and discuss evidence-based strategies for promoting healthy aging.
We will continue to guide you step by step through the complex process of aging, offering the best resources on preventive medicine and aging.
To health, vitality, and the years ahead!
Check out our website to book your personal healthy aging check!
We love hearing from you! Leave a comment below or share our post!
References
Aunan, J. R., Watson, M. M., Hagland, H. R., & Søreide, K. (2016). Molecular and biological hallmarks of ageing. British Journal of Surgery, 103(2), e29–e46. https://doi.org/10.1002/bjs.10053
Belsky, D. W., Huffman, K. M., Pieper, C. F., Shalev, I., & Kraus, W. E. (2017). Change in the Rate of Biological Aging in Response to Caloric Restriction: CALERIE Biobank Analysis. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 73(1), 4–10. https://doi.org/10.1093/gerona/glx096
Burch, J. B., Augustine, A. D., Frieden, L. A., Hadley, E., Howcroft, T. K., Johnson, R., Khalsa, P. S., Kohanski, R. A., Li, X. L., Macchiarini, F., Niederehe, G., Oh, Y. S., Pawlyk, A. C., Rodriguez, H., Rowland, J. H., Shen, G. L., Sierra, F., & Wise, B. C. (2014). Advances in Geroscience: Impact on Healthspan and Chronic Disease. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 69(Suppl 1), S1–S3. https://doi.org/10.1093/gerona/glu041
Campisi, J., Kapahi, P., Lithgow, G. J., Melov, S., Newman, J. C., & Verdin, E. (2019). From discoveries in ageing research to therapeutics for healthy ageing. Nature, 571(7764), 183–192. https://doi.org/10.1038/s41586-019-1365-2
Canudas, S., Becerra-Tomás, N., Hernández-Alonso, P., Galié, S., Leung, C., Crous-Bou, M., De Vivo, I., Gao, Y., Gu, Y., Meinilä, J., Milte, C., García-Calzón, S., Marti, A., Boccardi, V., Ventura-Marra, M., & Salas-Salvadó, J. (2020). Mediterranean Diet and Telomere Length: A Systematic Review and Meta-Analysis. Advances in Nutrition, 11(6), 1544–1554. https://doi.org/10.1093/advances/nmaa079
Chang, A. Y., Skirbekk, V. F., Tyrovolas, S., Kassebaum, N. J., & Dieleman, J. L. (2019). Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. The Lancet Public Health, 4(3), e159–e167. https://doi.org/10.1016/S2468-2667(19)30019-2
Chavda, V. P., Balar, P. C., Vaghela, D. A., & Dodiya, P. (2024). Unlocking longevity with GLP-1: A key to turn back the clock? Maturitas, 186, 108028. https://doi.org/10.1016/j.maturitas.2024.108028
Ducharme, J. (2023, November 30). Why Healthspan May Be More Important Than Lifespan. TIME. https://time.com/6341027/what-is-healthspan-vs-lifespan/
Fahy, G. M., Brooke, R. T., Watson, J. P., Good, Z., Vasanawala, S. S., Maecker, H., Leipold, M. D., Lin, D. T. S., Kobor, M. S., & Horvath, S. (2019). Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell, 18(6), e13028. https://doi.org/10.1111/acel.13028
Fazeli, P. K., Lee, H., & Steinhauser, M. L. (2020). Aging is a powerful risk factor for type 2 diabetes mellitus independent of body mass index. Gerontology, 66(2), 209–210. https://doi.org/10.1159/000501745
Ferrucci, L., & Fabbri, E. (2018). Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology, 15(9), 505–522. https://doi.org/10.1038/s41569-018-0064-2
Fleg, J. L., & Strait, J. (2012). Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Failure Reviews, 17(0), 545–554. https://doi.org/10.1007/s10741-011-9270-2
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C., & Santoro, A. (2018). Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology, 14(10), 576–590. https://doi.org/10.1038/s41574-018-0059-4
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., Ferrucci, L., Gilroy, D. W., Fasano, A., Miller, G. W., Miller, A. H., Mantovani, A., Weyand, C. M., Barzilai, N., Goronzy, J. J., Rando, T. A., Effros, R. B., Lucia, A., Kleinstreuer, N., & Slavich, G. M. (2019). Chronic inflammation in the etiology of disease across the life span. Nature Medicine, 25(12), 1822–1832. https://doi.org/10.1038/s41591-019-0675-0
Gladyshev, V. N., Kritchevsky, S. B., Clarke, S. G., Cuervo, A. M., Fiehn, O., de Magalhães, J. P., Mau, T., Maes, M., Moritz, R., Niedernhofer, L. J., Van Schaftingen, E., Tranah, G. J., Walsh, K., Yura, Y., Zhang, B., & Cummings, S. R. (2021). Molecular Damage in Aging. Nature Aging, 1(12), 1096–1106. https://doi.org/10.1038/s43587-021-00150-3
Guo, J., Huang, X., Dou, L., Yan, M., Shen, T., Tang, W., & Li, J. (2022). Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduction and Targeted Therapy, 7(1), 391. https://doi.org/10.1038/s41392-022-01251-0
Hoeijmakers, J. H. J. (2009). DNA Damage, Aging, and Cancer. New England Journal of Medicine, 361(15), 1475–1485. https://doi.org/10.1056/NEJMra0804615
Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., & Bohr, V. A. (2019). Ageing as a risk factor for neurodegenerative disease. Nature Reviews. Neurology, 15(10), 565–581. https://doi.org/10.1038/s41582-019-0244-7
Jurić, J., Kohrt, W. M., Kifer, D., Gavin, K. M., Pezer, M., Nigrovic, P. A., & Lauc, G. (2020). Effects of estradiol on biological age measured using the glycan age index. Aging, 12(19), 19756–19765. https://doi.org/10.18632/aging.104060
Kaeberlein, M. (2013). Longevity and aging. F1000prime Reports, 5, 5. https://doi.org/10.12703/P5-5
Kaeberlein, M. (2018). How healthy is the healthspan concept? GeroScience, 40(4), 361–364. https://doi.org/10.1007/s11357-018-0036-9
Kaeberlein, M. (2019). It is Time to Embrace 21st-Century Medicine. Public Policy & Aging Report, 29(4), 111–115. https://doi.org/10.1093/ppar/prz022
Kaeberlein, M., Rabinovitch, P. S., & Martin, G. M. (2015). Healthy aging: The ultimate preventative medicine. Science (New York, N.Y.), 350(6265), 1191–1193. https://doi.org/10.1126/science.aad3267
La Grotta, R., Frigé, C., Matacchione, G., Olivieri, F., de Candia, P., Ceriello, A., & Prattichizzo, F. (2022). Repurposing SGLT-2 Inhibitors to Target Aging: Available Evidence and Molecular Mechanisms. International Journal of Molecular Sciences, 23(20), 12325. https://doi.org/10.3390/ijms232012325
Larsson, L., Degens, H., Li, M., Salviati, L., Lee, Y. I., Thompson, W., Kirkland, J. L., & Sandri, M. (2019). Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiological Reviews, 99(1), 427–511. https://doi.org/10.1152/physrev.00061.2017
Lee, D. J. W., Kuerec, A. H., & Maier, A. B. (2024). Targeting ageing with rapamycin and its derivatives in humans: A systematic review. The Lancet Healthy Longevity, 5(2), e152–e162. https://doi.org/10.1016/S2666-7568(23)00258-1
Lee, E., Carreras-Gallo, N., Lopez, L., Turner, L., Lin, A., Mendez, T. L., Went, H., Tomusiak, A., Verdin, E., Corley, M., Ndhlovu, L., Smith, R., & Dwaraka, V. B. (2024). Exploring the effects of Dasatinib, Quercetin, and Fisetin on DNA methylation clocks: A longitudinal study on senolytic interventions. Aging, 16(4), 3088–3106. https://doi.org/10.18632/aging.205581
Lohman, T., Bains, G., Cole, S., Gharibvand, L., Berk, L., & Lohman, E. (2023). High-Intensity interval training reduces transcriptomic age: A randomized controlled trial. Aging Cell, 22(6), e13841. https://doi.org/10.1111/acel.13841
Lombard, D. B., Miller, R. A., & Pletcher, S. D. (2016). Biology of Aging and Longevity. In J. B. Halter, J. G. Ouslander, S. Studenski, K. P. High, S. Asthana, M. A. Supiano, & C. Ritchie (Eds.), Hazzard’s Geriatric Medicine and Gerontology (7th ed.). McGraw-Hill Education. accessmedicine.mhmedical.com/content.aspx?aid=1136585498
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The Hallmarks of Aging. Cell, 153(6), 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2023). Hallmarks of aging: An expanding universe. Cell, 186(2), 243–278. https://doi.org/10.1016/j.cell.2022.11.001
Lu, A. T., Fei, Z., Haghani, A., Robeck, T. R., Zoller, J. A., Li, C. Z., Lowe, R., Yan, Q., Zhang, J., Vu, H., Ablaeva, J., Acosta-Rodriguez, V. A., Adams, D. M., Almunia, J., Aloysius, A., Ardehali, R., Arneson, A., Baker, C. S., Banks, G., … Horvath, S. (2023). Universal DNA methylation age across mammalian tissues. Nature Aging, 1–23. https://doi.org/10.1038/s43587-023-00462-6
Lu, Y. R., Tian, X., & Sinclair, D. A. (2023). The Information Theory of Aging. Nature Aging, 3(12), Article 12. https://doi.org/10.1038/s43587-023-00527-6
Mahmoudi, S., Xu, L., & Brunet, A. (2019). Turning back time with emerging rejuvenation strategies. Nature Cell Biology, 21(1), 32–43. https://doi.org/10.1038/s41556-018-0206-0
Maresova, P., Javanmardi, E., Barakovic, S., Barakovic Husic, J., Tomsone, S., Krejcar, O., & Kuca, K. (2019). Consequences of chronic diseases and other limitations associated with old age – a scoping review. BMC Public Health, 19, 1431. https://doi.org/10.1186/s12889-019-7762-5
Mattace-Raso, F. U. S., van der Cammen, T. J. M., Hofman, A., van Popele, N. M., Bos, M. L., Schalekamp, M. A. D. H., Asmar, R., Reneman, R. S., Hoeks, A. P. G., Breteler, M. M. B., & Witteman, J. C. M. (2006). Arterial Stiffness and Risk of Coronary Heart Disease and Stroke. Circulation, 113(5), 657–663. https://doi.org/10.1161/CIRCULATIONAHA.105.555235
Meyer, D. H., & Schumacher, B. (2024). Aging clocks based on accumulating stochastic variation. Nature Aging, 4(6), 871–885. https://doi.org/10.1038/s43587-024-00619-x
Moqri, M., Herzog, C., Poganik, J. R., Ying, K., Justice, J. N., Belsky, D. W., Higgins-Chen, A. T., Chen, B. H., Cohen, A. A., Fuellen, G., Hägg, S., Marioni, R. E., Widschwendter, M., Fortney, K., Fedichev, P. O., Zhavoronkov, A., Barzilai, N., Lasky-Su, J., Kiel, D. P., … Ferrucci, L. (2024). Validation of biomarkers of aging. Nature Medicine, 1–13. https://doi.org/10.1038/s41591-023-02784-9
Niccoli, T., & Partridge, L. (2012). Ageing as a Risk Factor for Disease. Current Biology, 22(17), R741–R752. https://doi.org/10.1016/j.cub.2012.07.024
O’Keefe, J. H., Weidling, R., O’Keefe, E. L., & Franco, W. G. (2023). SGLT inhibitors for improving Healthspan and lifespan. Progress in Cardiovascular Diseases, 81, 2–9. https://doi.org/10.1016/j.pcad.2023.10.003
Our World in Data. (2019). Probability of dying, by age. Our World in Data. https://ourworldindata.org/grapher/probability-of-dying-by-age?time=2019&facet=metric
Our World in Data. (2021). Depressive disorders prevalence, by age. Our World in Data. https://ourworldindata.org/grapher/depressive-disorders-prevalence-by-age
Partridge, L., Deelen, J., & Slagboom, P. E. (2018). Facing up to the global challenges of ageing. Nature, 561(7721), 45–56. https://doi.org/10.1038/s41586-018-0457-8
Sánchez-González, J. L., Sánchez-Rodríguez, J. L., Varela-Rodríguez, S., González-Sarmiento, R., Rivera-Picón, C., Juárez-Vela, R., Tejada-Garrido, C. I., Martín-Vallejo, J., & Navarro-López, V. (2024). Effects of Physical Exercise on Telomere Length in Healthy Adults: Systematic Review, Meta-Analysis, and Meta-Regression. JMIR Public Health and Surveillance, 10, e46019. https://doi.org/10.2196/46019
Selvarani, R., Mohammed, S., & Richardson, A. (2020). Effect of rapamycin on aging and age-related diseases—Past and future. GeroScience, 43(3), 1135–1158. https://doi.org/10.1007/s11357-020-00274-1
TAME - Targeting Aging with Metformin. (2024). American Federation for Aging Research. https://www.afar.org/tame-trial
The Global Health Observatory. (2019). Life expectancy and healthy life expectancy. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy
Waziry, R., Ryan, C. P., Corcoran, D. L., Huffman, K. M., Kobor, M. S., Kothari, M., Graf, G. H., Kraus, V. B., Kraus, W. E., Lin, D. T. S., Pieper, C. F., Ramaker, M. E., Bhapkar, M., Das, S. K., Ferrucci, L., Hastings, W. J., Kebbe, M., Parker, D. C., Racette, S. B., … Belsky, D. W. (2023). Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults from the CALERIE trial. Nature Aging, 3(3), Article 3. https://doi.org/10.1038/s43587-022-00357-y
Wen, J., Tian, Y. E., Skampardoni, I., Yang, Z., Cui, Y., Anagnostakis, F., Mamourian, E., Zhao, B., Toga, A. W., Zalesky, A., & Davatzikos, C. (2024). The genetic architecture of biological age in nine human organ systems. Nature Aging, 1–18. https://doi.org/10.1038/s43587-024-00662-8
WHO, W. H. O. (2022). Ageing and health. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
Yan, M., Sun, S., Xu, K., Huang, X., Dou, L., Pang, J., Tang, W., Shen, T., & Li, J. (2021). Cardiac Aging: From Basic Research to Therapeutics. Oxidative Medicine and Cellular Longevity, 2021, 9570325. https://doi.org/10.1155/2021/9570325